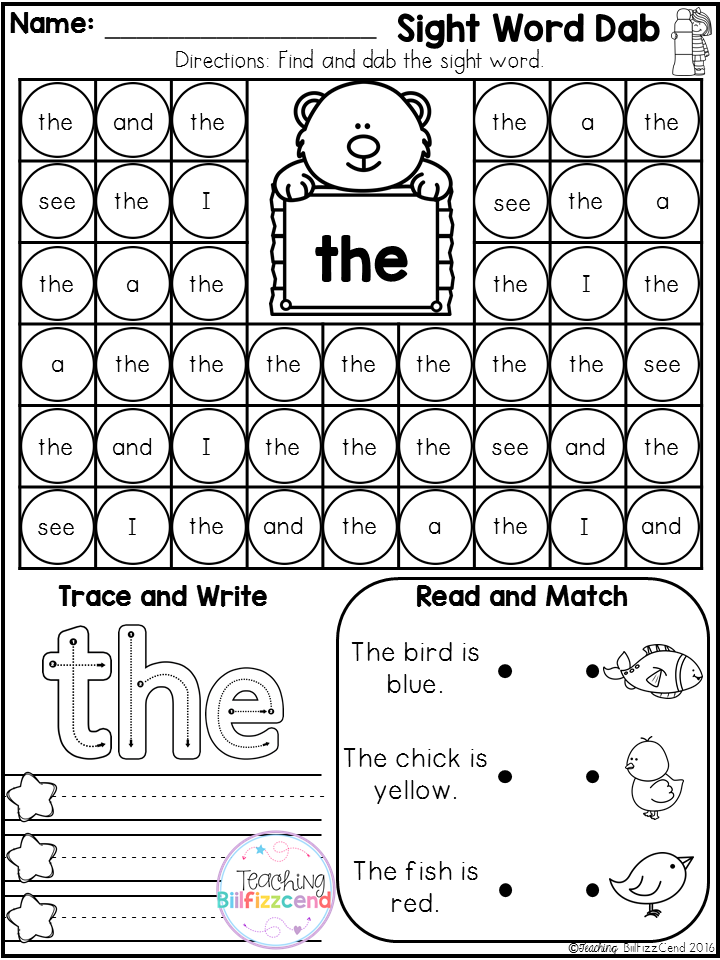
Alphabetic principle activities
Alphabet Matching | Classroom Strategies
Very young learners are developing their understanding of the alphabetic principle — the understanding that there are systematic and predictable relationships between written letters and spoken sounds. Teachers can help students develop this understanding through lots of fun activities that help students explore the alphabet letters and sounds.
| How to use: | Individually | With small groups | Whole class setting |
More phonics strategies
Why teach about the alphabet?
- Letter naming is a strong predictor of later reading success
- Learning letter names helps a child learn letter sounds
- It helps students develop their understanding of the alphabetic principle
Watch: Alphabet In My Mouth!
Students practice each letter-name, sound, and corresponding action to help solidify letter-sound correspondences in an active and engaging way — with a song. See the lesson plan.
This video is published with permission from the Balanced Literacy Diet. See many more related how-to videos with lesson plans in the Letter-Sounds and Phonics section.
Collect resources
Matching upper-case and lower-case
Teachers can use the following activity to ask students to help the "Mama animals" (uppercase letters) find their "babies" (lowercase letters). This game includes matching the uppercase mothers with their lowercase babies. See example >
This link provides a template for a printable "Superhero" upper- and lowercase letter match game. See example >
This file includes uppercase and lowercase letters in a matching game that parents can use with their child at home. See example >
This link provides templates for printing cards to use for writing uppercase and lowercase letters. See example >
See example >
Letter formation: using sand, play dough, or flour
This link provides teachers with downloadable mats with the alphabet letters for helping children use play dough for learning letter formation. See example >
Letter bingo
Bingo is a simple game that children enjoy and can be used to help them learn about the upper- and lowercase letters. This link allows teachers to print the letters and board needed to play letter bingo. See example >
Letter stamps
Stamps are an excellent "hands-on" activity for helping students learn about the alphabet. The activity described in the link below provides teachers with some creative ideas for making letter stamps out of sponges. Teachers can use sponges and paint in a variety of ways to help children understand the shape and function of upper- and lowercase letters. See example >
Letter recognition fluency
This online document contains several activities that are helpful for building letter recognition fluency.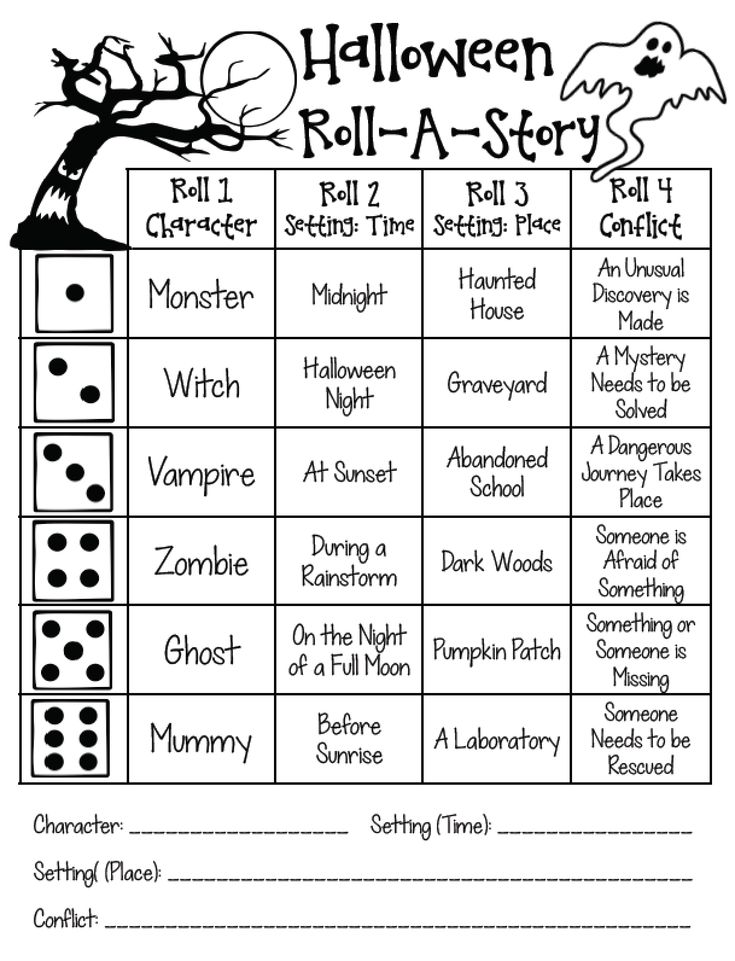 Teachers can download materials needed and follow the instructions for each activity. There are also some ideas included for extending and adapting each activity to further enhance learning. Some examples are provided below. Download activities >
Teachers can download materials needed and follow the instructions for each activity. There are also some ideas included for extending and adapting each activity to further enhance learning. Some examples are provided below. Download activities >
Speedy Alphabet Arc: Teachers can download and print a copy of the alphabet "arc" and have students use letters to match the ones on the arc. Parents could use this idea at home with magnetic letters by placing the arc on the refrigerator and have the child match the letters. Using a timer and seeing how quickly the child can match the letters is optional.
Glow Go: This activity includes the use of glow in the dark chalk and black construction paper. Students can work together taking turns using a flashlight and naming the letters.
Hungry Letter Mouse: Teachers can utilize this activity for students to work on letter recognition in pairs using an eraser and dry erase marker.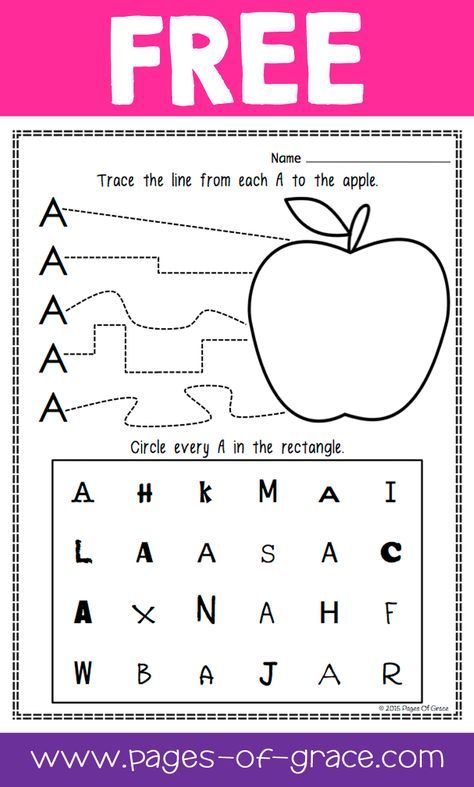 One student can use the eraser to be the mouse and the other student names the letter before the "mouse" eats it (i.e., erases it).
One student can use the eraser to be the mouse and the other student names the letter before the "mouse" eats it (i.e., erases it).
Letter books
Teachers can use the downloadable materials form this website to create letter books in which each page contains one letter. The kids draw or cut pictures from magazines that start with the particular letter and glue them into their "book." See example >
Downloadable letter and alphabet cards
Flash cards
The link listed below provides teachers with downloadable ESL flashcards. These are free and printable for use within the classroom. There are large sets for use with teaching letters and vocabulary, and smaller sets for language learning games. See example >
This website provides printable color flashcards that are great for teaching upper- and lowercase letters. These cards are free and use the Zaner Bloser font which is very simplistic — ideal for teaching young children.
See example >
">
Alphabet cards
The downloadable alphabet cards available from this link have various fonts to choose from, color picture cards to accompany the letters, and creative ideas for activities. See example >
See example >
Race track alphabet
The website below offers teachers the ability to download letters of the alphabet in race-track format. Children can use toy cars to trace around the letters to help learn the formation. See example >
Differentiated instruction
for Second Language Learners, students of varying reading skill, and for younger learners
- Begin with a very simple, plain alphabet font like Zaner Bloser. As children become more familiar with letter shapes, progress to different fonts that may present letters slightly differently. For example, consider how the letters /a/, /g/, /t/ appear in different fonts.
- Decide how many letters a child or group should work with at one time. Very emergent learners should begin with fewer letters; other students can manage working with more letters at one time.
- Just for fun! Serve alphabet soup or use Alpha-bits cereal as an extra reinforcement of letters.
See the research that supports this strategy
Adams, M. (1990). Beginning to read: Thinking and learning about print. Cambridge, MA: MIT Press.
(1990). Beginning to read: Thinking and learning about print. Cambridge, MA: MIT Press.
Snow, C., Burns, M., & Griffin, P. (Eds.). (1998). Preventing reading difficulties in young children. Washington, DC: National Academy Press.
Texas Education Agency. (2002). The Alphabetic Principle.
Children's books to use with this strategy
Farm Alphabet Book
By: Jane Miller
Genre: Nonfiction
Age Level: 0-3
Reading Level: Pre-Reader
The alphabet is presented in upper and lower case letters accompanied by full color photographs that introduce farms and things associated.
Kipper's A to Z: An Alphabet Adventure
By: Nick Inkpen
Age Level: 0-3
Reading Level: Pre-Reader
Upper and lower case letters, clearly printed, are introduced by Kipper and Arnold in a playful, imaginative tale plainly intended as an alphabet book.
Eating the Alphabet
By: Lois Ehlert
Genre: Fiction
Age Level: 0-3
Reading Level: Pre-Reader
Clean lines of both upper and lower case letters combine with colorful fruits and vegetables for a unique way to think about - and even eat through the alphabet.
Chicka Chicka Boom Boom
By: Bill Martin Jr, John Archambault
Genre: Fiction
Age Level: 0-3
Reading Level: Pre-Reader
Naughty lowercase letters climb the coconut tree but when little Z gets to the top, they all go BOOM to the bottom. After a rescue by grown-up letters (all uppercase), it all seems to start again. Humor, crisp illustration and rhythm make this alphabetic adventure a classic.
ABC: A Child's First Alphabet
By: Allison Jay
Age Level: 0-3
Reading Level: Pre-Reader
Upper and lower case letters introduce familiar objects (e.g., apple) while full page illustrations depict both obvious (and less so) objects that begin with the same letter.
Comments
Alphabetic Principle Activities - Ms. McEvoy
Description: The teacher prints out the cards (one set for each student) and has them cut up the cards. Once they are all
cut up the students try to match the baby animal (lower case) to the mama animal (upper case) for each letter of the alphabet.
Reading Component: This activity focuses on a student’s ability to identify upper
and lower case letters in the alphabet and also ties in the animals that start with that letter.
Skill Level: This activity would be best used in a preschool or pre-k classroom when students are first being introduced to letters and their forms.
Example of how to use strategy: This activity is best used to introduce letters and to reinforce how every letter has a lowercase and uppercase form.
Strengths and Weaknesses of strategy: A strength of this activity is that it is a fun way for students to learn about upper and lowercase letters by matching up animals. They are learning without really being aware of how important what they are learning is. There are no weaknesses of using this activity in the classroom.
Information gained from this activity for formative assessment or IEP goals: The information gained from this activity is helpful in knowing where a student is with understanding alphabetic principle. If they are struggling then it is important for the IEP team to focus on strategies for the student to master that skill. If a student is really grasping the information then it is important for them to move on to more advanced skills.
If they are struggling then it is important for the IEP team to focus on strategies for the student to master that skill. If a student is really grasping the information then it is important for them to move on to more advanced skills.
Activity: Family Pairs
http://www.readinga-z.com/books/alphabet-books/strategy-bank/
Description: Teacher writes word family pairs on the board and has students identify what letters are different in each pair and then asks if the students can name and initial letter that makes another word.
Reading Component: This activity focuses on students’ understanding of letter conventions and how they play into a word’s meaning. It challenges students to think about letters to change a word.
Skill Level: This activity would be best used in a kindergarten or first grade classroom while students are still learning spelling and how to use letters.
Example of how to use strategy: Word pairs that can be used are:
cat — bat
sat — fat
dog — hog
tag — sag
mad — bad
lot — pot
see — bee
man — can
hit — sit
pit — bit
bow — low
cow — vow
Strengths and Weaknesses of strategy: A strength of this activity is that it engages students to think freely about new words they can come up with and how different letters change the words on the board. A weakness of this activity is classroom management, since it is an activity that requires feedback students may begin to shout out and interrupt each other.
A weakness of this activity is classroom management, since it is an activity that requires feedback students may begin to shout out and interrupt each other.
Information gained from this activity for formative assessment or IEP goals: If a student is really struggling with understanding how to do this activity and cannot come up with words or letters to change the words it may be a sign that they need extra support in phonological awareness as well as alphabetic understanding which can be worked into their IEP goals.
Activity: Alphabet Bingo
http://www.readingrockets.org/content/pdfs/AlphaBingo.pdf
Description: This activity is bingo, but it uses upper and lower case letters. The teacher prints out the bingo cards then will say a letter and have the students mark it on their cards to see who gets bingo first.
Reading Component: By playing bingo with upper and lower case letters it reinforces understanding of the different forms a letter can have and how it represents the same sounds.
Skill Level: This activity would be used with younger students in pre-k or kindergarten.
Example of how to use strategy: You would use this activity in pre-k or kindergarten after students learned about letters as a way to practice.
Strengths and Weaknesses of strategy: A strength of this activity is that it turns practice of alphabetic principle into a game that is fun for students. Another strength is the teacher can write the opposite form on the board and have the students look for that same letter (in the other form), say the letter name, or make the letter sound to practice different elements of alphabetic principle with students.
Information gained from this activity for formative assessment or IEP goals: A teacher can gain information on how much of the alphabet a student is grasping based off of this activity as well as what letters they are struggling with.
Activity: Building Words
http://www.bluevalleyk12.org/education/components/scrapbook/default.php?sectiondetailid=61313
Description: This activity is designed to be used at home, but can be used in the classroom as well. In the classroom the teacher uses magnetic letters on a board to form a word using only a few letters, then each day the teacher changes one letter to turn it into a different word and so on to practice how different letters change a word to mean something else.
Reading Component: This activity incorporates alphabetic decoding and recoding by having students focus on how a word changes based on what letters are added, taken away, or changed.
Skill Level: You would use this activity in either kindergarten, first, or second grade once students have an understanding of letters and what sounds they make.
Example of how to use strategy: Example sequence could be
hat
bat
bar
car
card
cart
mart
mark
bark
bare
care
fare
far
fat
pat
par
Strengths and Weaknesses of strategy: A strength of this activity is that it challenges students to think about words by each of their letters and how to change them based on a letter or two.
Information gained from this activity for formative assessment or IEP goals: This activity doesn't provide too much information that directly impacts assessment or IEP goals, but it can be a fun way to see where students are at with alphabetic understanding.
Activity: Letter I Spy
http://www.readinga-z.com/books/alphabet-books/strategy-bank/
Description: Teacher has students sit at their desks or in a circle on the floor and plays I Spy with them by describing objects around the room; example “I spy something that begins with B. you can read it” for a book.
Reading Component: This activity helps students with real world application of how letters correspond with sounds that correspond with words and meanings.
Skill Level: This activity would most likely be used in a kindergarten or first grade classroom because it is a relatively simple activity.
Example of how to use strategy: This game can be played with little to no planning, all the teacher has to do is get creative with what they choose to say and have the students look for.
Strengths and Weaknesses of strategy: A strength of this activity is that it is easy to do and can be very fun for students, but a weakness is that it isn't individualized to target specific students, just the class as a whole.
Information gained from this activity for formative assessment or IEP goals: Unfortunately, this activity doesn't provide individual feedback on how specific students are doing so it isn't the best to use for assessment or IEP goal information.
The Ministry of Health and Social Development of the Russian Federation clarified the issues of storage of medicines that arise for legal entities and individual entrepreneurs
- Main
- Legal resources
- "Hot" documents
- Information letter of the Ministry of Health and Social Development of the Russian Federation dated February 8, 2011 N 25-1/10/2-1208 "On the storage of medicines"
Information letter of the Ministry of Health and Social Development of the Russian Federation dated February 8, 2011 N 25-1/10/2-1208
In particular, it is reported that the Rules for the storage of medicines, approved by the Order of the Ministry of Health and Social Development of the Russian Federation of August 23, 2010 N 706n, establish requirements for storage facilities for medicines for medical use and regulate the conditions for their storage.
The Rules establish methods for the placement of medicines. When placing medicines, it is allowed to use computer technologies (in alphabetical order, by codes). The Rules do not provide for the need for an organization that stores medicines using computer technology to obtain permission from any state authority for such a method of storing medicines.
The Rules define the need to identify stored medicines (regardless of where they are stored) either using a rack card or, using computer technology, using codes and electronic devices. According to the Ministry of Health and Social Development of the Russian Federation, it is advisable to fix the chosen systematization of storage of medicines by order of the head of a legal entity or an individual entrepreneur.
In accordance with the Rules, packaged medicinal herbal raw materials are stored on racks or in cabinets. There are no other requirements for the storage of this group of medicinal products. Thus, for the storage of packaged medicinal plant materials (in primary and secondary packaging), it is not necessary to allocate separate zones or rooms.
The requirements for separate storage (in a closet or room) apply only to bulk medicinal plant materials that are potent or toxic substances in accordance with Decree of the Government of the Russian Federation of December 29, 2007 N 964 "On approval of lists of potent and toxic substances for the purposes of Article 234 and other articles of the Criminal Code of the Russian Federation, as well as a large amount of potent substances for the purposes of Article 234 of the Criminal Code of the Russian Federation.
The Rules establish a norm for the storage of potent and poisonous drugs under international control in premises equipped with engineering and technical security equipment similar to those provided for the storage of narcotic and psychotropic drugs.
This rule applies to potent and toxic substances - monopreparations under international control in accordance with the UN Convention on Psychotropic Substances 1971 and the United Nations Convention for the Suppression of Illicit Traffic in Narcotic Drugs and Psychotropic Substances of 1988.
More documents and clarifications on anti-crisis measures - in the ConsultantPlus system.
Register and get test access to
Date of publication on the site: 03/16/2011
Share link:
90,000 storage of drugs 1.1.0010.15
Burning0016
Storage of medicines GPM.1.1.0010.15
Introduced for the first time
This general monograph establishes general requirements for the storage of pharmaceutical substances, excipients and medicinal products and applies to all organizations in which medicinal products are stored, taking into account the type of activity of the organization.
Storage of medicinal herbal raw materials and herbal medicinal products is carried out in accordance with the General Pharmacopoeia Monograph “Storage of medicinal herbal raw materials and medicinal herbal preparations”.
Storage - the process of storing medicinal products until they are used within the established expiration date, which is an integral part of the circulation of medicinal products.
General requirements for storage facilities
medicines and organization of their storage
Storage of medicines must be carried out in the premises intended for these purposes. The device, composition, size of storage areas, their operation and equipment should ensure proper storage conditions for various groups of medicines.
The complex of premises for storage should include:
- a room (area) for acceptance intended for unpacking and receiving packages of medicines and their preliminary examination;
- room (area) for sampling medicines in accordance with the requirements of the General Pharmacopoeia Monograph "Sampling";
- premises (zone) for quarantine storage of medicines;
- premises for medicines requiring special storage conditions;
- a room (area) for storing rejected, returned, recalled and / or expired medicines. These medicinal products and their places of storage must be clearly marked.
A storage area is allocated in a common storage room in the absence of a separate isolated room.
Finishing of premises for the storage of medicines must meet current sanitary and hygienic requirements, the internal surfaces of walls and ceilings must be smooth, allowing the possibility of wet cleaning.
In each storage room, it is necessary to maintain a climate regime, observing the temperature and humidity of the air established by the monograph or regulatory documentation for medicinal products. The necessary air exchange in the storage rooms is created using air conditioners, supply and exhaust ventilation or other equipment. Natural and artificial lighting in storage rooms must ensure that all operations carried out in the room are carried out accurately and safely. If necessary, the protection of medicines from solar radiation should be provided.
Premises for the storage of medicines must be equipped with the necessary number of measuring instruments (thermometers, hygrometers, psychrometers, etc. ) certified in accordance with the established procedure for monitoring and recording temperature and humidity, carried out at least once a day. Measuring instruments are placed at a distance of at least 3 m from doors, windows and heaters in a place accessible for reading readings, at a height of 1.5 - 1.7 m from the floor. At the same time, they are recommended to be placed in places where there is the greatest probability of temperature and humidity fluctuations or deviations from the required parameters are most often observed.
) certified in accordance with the established procedure for monitoring and recording temperature and humidity, carried out at least once a day. Measuring instruments are placed at a distance of at least 3 m from doors, windows and heaters in a place accessible for reading readings, at a height of 1.5 - 1.7 m from the floor. At the same time, they are recommended to be placed in places where there is the greatest probability of temperature and humidity fluctuations or deviations from the required parameters are most often observed.
Registration records must show the temperature and humidity regimes established for the premises, and, if they do not comply, corrective actions.
Storage rooms must be equipped with a sufficient number of cabinets, safes, shelving, storage boxes, pallets. Equipment must be in good condition and clean.
Shelving, cabinets and other equipment must be installed in such a way as to provide access to medicines, free passage of personnel and, if necessary, accessibility of loading and unloading operations, as well as accessibility of equipment, walls, floors of the cleaning room.
Premises for the storage of medicines must be maintained in a proper sanitary regime. The frequency and methods of cleaning the premises must comply with the requirements of regulatory documents. Sanitary disinfectants used must be safe, and the risk of contaminating stored medicinal products with these products must be excluded.
Special instructions must be in place for cleaning up spilled or spilled medicinal products in order to completely eliminate and prevent contamination of other medicinal products.
Employees must wear special clothing and footwear while performing work in the premises for the storage of medicines, and observe the rules of personal hygiene.
Medicinal products are placed in storage rooms in accordance with the storage conditions specified in the pharmacopoeial monograph or regulatory documentation for medicinal products, taking into account their physicochemical and hazardous properties, pharmacological and toxicological effects, type of dosage form of the medicinal product and method of its application , state of aggregation of the medicinal product. When using computer technology, it is allowed to place medicines alphabetically, by codes.
When using computer technology, it is allowed to place medicines alphabetically, by codes.
Shelves, cabinets, shelves intended for storage of medicines must be identified. It is also necessary to identify stored medicines using a rack card, using computer technology - using codes and electronic devices.
In the case of manual loading and unloading operations, the stacking height of medicinal products should not exceed 1.5 m. When using mechanized devices, medicinal products should be stored in several tiers during unloading and loading operations. At the same time, the total height of the placement of medicines on the racks should not exceed the capabilities of the loading and unloading mechanisms.
Medicinal products in storage rooms should be placed in cabinets, on racks, pedestals, pallets, etc. It is not allowed to place medicines on the floor without a pallet. Pallets can be placed on the floor in one row or on racks in several tiers, depending on the height of the rack. It is not allowed to place pallets with medicines in several rows in height without the use of racks.
It is not allowed to place pallets with medicines in several rows in height without the use of racks.
When creating storage conditions for a single medicinal product, it is necessary to be guided by the requirements specified in the pharmacopoeial monograph or regulatory documentation for this medicinal product, established by the manufacturer (developer) of the medicinal product based on the results of stability studies in accordance with the General Pharmacopoeia Monograph "Expiry date of medicinal products".
Storage of medicinal products is carried out in packaging (consumer, group) that meets the requirements of the regulatory documentation for this medicinal product.
Storage of medicinal products is carried out at a relative humidity of not more than 60 ± 5%, depending on the corresponding climatic zone (I, II, III, IVA, IVB), if special storage conditions are not specified in the regulatory documentation.
Medicines should be stored in a way that prevents contamination, mixing and cross-contamination. Extraneous odors in storage rooms should be avoided.
Extraneous odors in storage rooms should be avoided.
The organization's established accounting system for medicines with a limited shelf life should be implemented. If several batches of the same name of the medicinal product are in storage, then the medicinal product, the expiration date of which expires earlier than the others, should be taken first for use.
Rejected medicinal products must be identified and stored in an appropriate room (area) in conditions that do not allow their unauthorized use.
Features of storage of certain groups of medicines
Medicines with hazardous properties (flammable, explosive, radiopharmaceutical, caustic, corrosive, compressed and liquefied gases, etc.) should be stored in specially arranged rooms equipped with additional safety equipment and protection. During storage, it is necessary to ensure the safety and declared quality of medicinal products, prevent the possibility of manifestation of their dangerous properties by medicinal products and create safe working conditions for employees working with such medicinal products.
When arranging premises and organizing the storage of hazardous medicines, it is necessary to be guided by the requirements of federal laws and regulatory legal acts of the Russian Federation.
Storage of narcotic and psychotropic drugs must be carried out in accordance with federal laws and regulations of the Russian Federation.
When storing medicines that require protection from the influence of environmental factors (light, temperature, atmospheric composition of air, etc.), it is necessary to ensure the storage regime specified in the pharmacopoeial monograph or regulatory documentation. Deviations from the regulated conditions are allowed once only for a short period (no more than 24 hours), unless special conditions, for example, permanent storage in a cold place, are specified separately.
Medicines that can change their properties (oxidize, reduce, decompose, change their color, etc.) under the action of light energy are photo- or light-sensitive; light-resistant drugs are photostable. The influence of light energy can be manifested in the impact of direct sunlight, scattered light in the visible region of the light spectrum and radiation in the ultraviolet region.
The influence of light energy can be manifested in the impact of direct sunlight, scattered light in the visible region of the light spectrum and radiation in the ultraviolet region.
Photosensitive medicinal products are usually labeled with the statement: “Keep protected from light”. Medicinal products requiring protection from light should be stored in rooms or specially equipped areas that provide protection from natural and artificial lighting. Pharmaceutical substances that require protection from light should be stored either in packages made of light-shielding materials or in a dark room or cabinets. If glass containers for medicines are used as packaging for pharmaceutical substances that are especially sensitive to light, the container must be pasted over with black opaque paper.
Photosensitive medicinal products must be packaged in light-protective secondary (consumer) packaging and/or must be stored in a place protected from light.
Medicines which, in contact with water, moisture, may release gases, etc.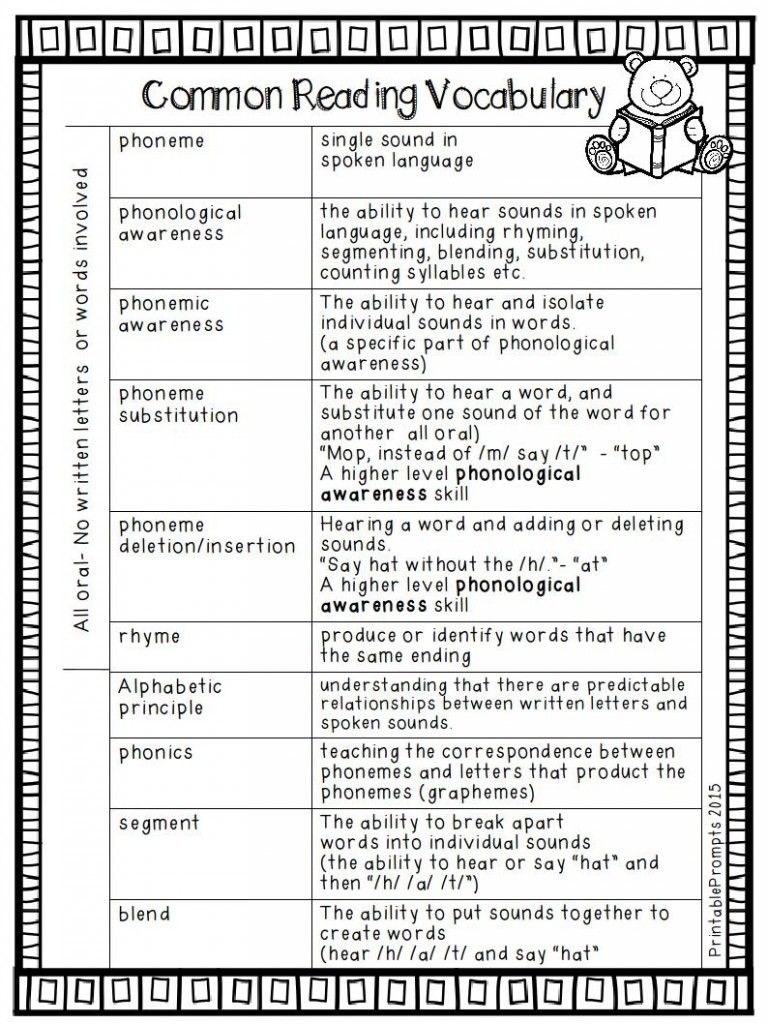 , are moisture sensitive. Moisture-sensitive medicinal products are usually labeled with the statement: “Keep dry”. When storing such medicinal products, it is necessary to create conditions so that the relative humidity of the air does not exceed 50% at room temperature (under normal storage conditions) or an equivalent vapor pressure at a different temperature. Compliance with the requirement also provides for the storage of a moisture-sensitive medicinal product in airtight (moisture-tight) consumer packaging that provides the specified protection and compliance with storage conditions when handling the medicinal product.
, are moisture sensitive. Moisture-sensitive medicinal products are usually labeled with the statement: “Keep dry”. When storing such medicinal products, it is necessary to create conditions so that the relative humidity of the air does not exceed 50% at room temperature (under normal storage conditions) or an equivalent vapor pressure at a different temperature. Compliance with the requirement also provides for the storage of a moisture-sensitive medicinal product in airtight (moisture-tight) consumer packaging that provides the specified protection and compliance with storage conditions when handling the medicinal product.
To maintain a low moisture content during storage of medicines, desiccants are used in prescribed cases, provided that they do not come into direct contact with the medicine.
Medicines with hygroscopic properties must be stored at a relative humidity of not more than 50% in a package that is a glass container for medicines, hermetically sealed, or in a package with additional protection, for example, in a bag of polyethylene film, in accordance with the requirements of the Pharmacopoeia articles or normative documents.
Some groups of drugs change their properties under the influence of atmospheric gases such as oxygen or carbon dioxide. To ensure the protection of medicines from the effects of gases, storage of medicines is recommended to be carried out in sealed packaging made of materials that are not permeable to gases. Packaging, if possible, should be filled to the top and sealed tightly.
Medicinal products which are volatile medicinal products per se or medicinal products containing a volatile solvent; solutions and mixtures of volatile substances; drugs that decompose with the formation of volatile products require storage conditions that protect them from volatilization and drying. It is recommended to store medicines in a cool place, in hermetically sealed packaging made of materials impervious to volatile substances or in primary and secondary (consumer) packaging in accordance with the requirements specified in the monograph or regulatory documentation.
Medicines, which are pharmaceutical substances containing water of crystallization (crystal hydrates), exhibit the properties of hygroscopic substances. Storage of crystalline hydrates is recommended to be carried out in hermetically sealed packaging in accordance with the requirements specified in the monograph or regulatory documentation. As a rule, crystalline hydrates are stored at a temperature of 8 to 15 °C and a relative air humidity of not more than 60%.
Storage of crystalline hydrates is recommended to be carried out in hermetically sealed packaging in accordance with the requirements specified in the monograph or regulatory documentation. As a rule, crystalline hydrates are stored at a temperature of 8 to 15 °C and a relative air humidity of not more than 60%.
Drugs that change their properties under the influence of ambient temperature are heat-sensitive. Drugs can change their properties under the influence of room and higher temperatures (thermolabile drugs) or under the influence of low temperatures, including freezing.
When storing heat-sensitive medicinal products, it is necessary to ensure the temperature regime regulated by the requirements of the pharmacopoeia monograph or regulatory documentation, indicated on the primary and / or secondary (consumer) packaging of the medicinal product.
Heat-labile drugs should be stored in specially equipped rooms (refrigerators) or in storage rooms equipped with a sufficient number of refrigerators, refrigerators. For the storage of thermolabile drugs, pharmaceutical refrigerators or refrigerators for blood and its products should be used.
For the storage of thermolabile drugs, pharmaceutical refrigerators or refrigerators for blood and its products should be used.
Proper quality of immunobiological medicinal products, safety and efficacy of their use is ensured by the cold chain system, which must be carried out at all its four levels.
Refrigerators (chambers, cabinets) must be set at a temperature corresponding to the temperature regime of storage of medicinal products contained in them. Storage of immunobiological medicinal products should be carried out at a temperature not exceeding 8 °C. Cooled air must be provided to each package of the immunobiological medicinal product in the refrigerator. Joint storage of immunobiological medicinal products with other medicinal products in the refrigerator is not allowed.
To monitor the temperature regime of storage of thermolabile medicinal products, all refrigerators (chambers, cabinets) must be provided with thermometers. Continuous monitoring of the temperature regime is carried out using thermographs and temperature recorders, the readings of which are recorded at least twice a day.
The temperature regime on the shelves of the refrigerator is different: the temperature is lower near the freezer, higher - near the opening door panel.
Provision of a cold place means storing drugs in a refrigerator at a temperature of 2 to 8 ° C, avoiding freezing. Cool storage refers to the storage of medicines at temperatures between 8 and 15°C. In this case, it is allowed to store medicines in a refrigerator, with the exception of medicines that, when stored at a refrigerator temperature below 8 ° C, can change their physical and chemical characteristics, for example, tinctures, liquid extracts, etc. Storage at room temperature implies temperature mode from 15 to 25 °С or, depending on climatic conditions, up to 30 °С. Storage in the freezer ensures the temperature regime of medicines from -5 to -18 °C. Storage under deep freezing conditions provides for a temperature regime below -18 ° C.
Medicines should be placed in areas and on shelves of the refrigerator, corresponding to their temperature storage conditions. Do not store immunobiological medicinal products on the door panel of the refrigerator.
Do not store immunobiological medicinal products on the door panel of the refrigerator.
In the storage rooms, it is necessary to provide storage conditions for medicines that require protection from exposure to low temperatures, for which the lower limit of the temperature storage regime is established in the pharmacopoeial monograph or regulatory documentation.
It is not allowed to freeze medicines that have the relevant requirements in the monograph or regulatory documentation and are indicated on the primary or secondary packaging, including insulin preparations, adsorbed immunobiological preparations, etc.
It is not allowed to freeze medicines placed in the package , capable of being destroyed by freezing, for example, drugs in ampoules, glass vials, etc.
The definitions used in the pharmacopoeia that characterize the temperature regimes for storing medicines are given in the table.
It is necessary to ensure compliance with the storage conditions of medicines and maintain their integrity during transportation.